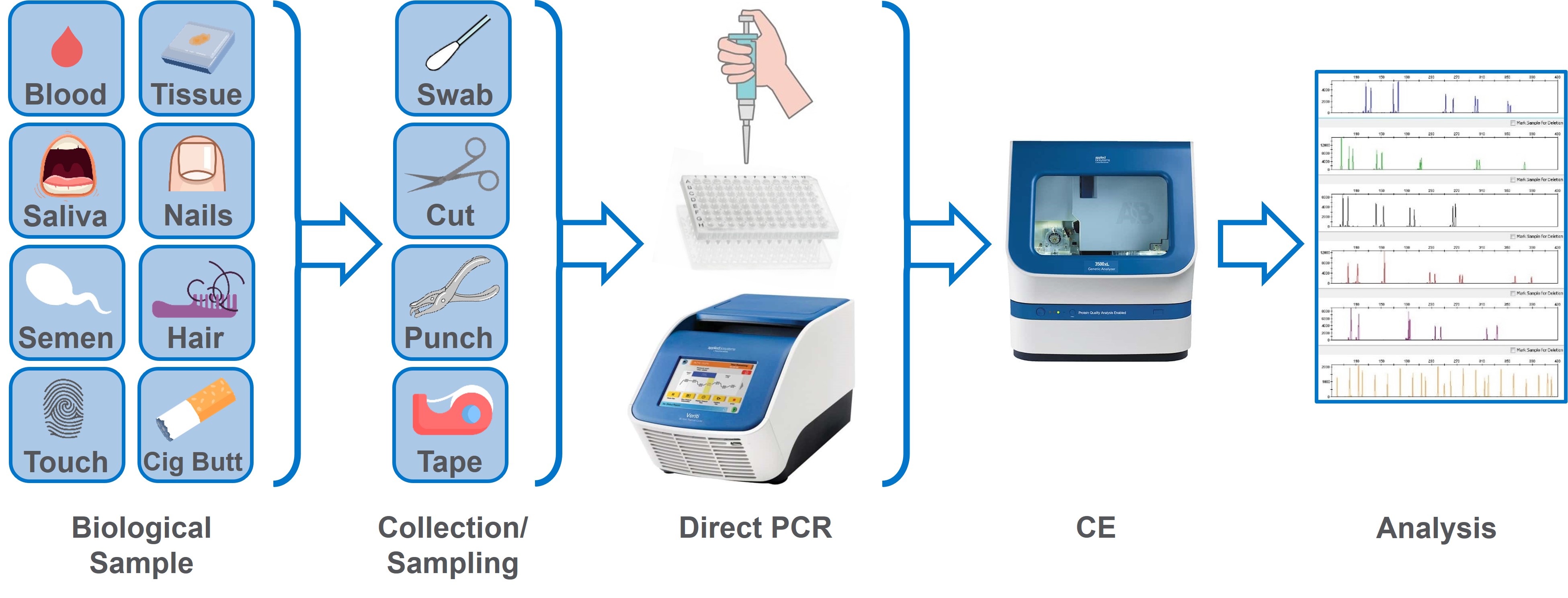
Sometimes forensic labs can find ample DNA in the evidence collected at a crime scene. Other times, investigators are not as fortunate. Evidence samples with low amounts of DNA may not yield a profile that investigators can use to match or exclude potential suspects.
Researchers have a potential solution: direct polymerase chain reaction (PCR). This DNA amplification method allows scientists to add a swab or sample directly to the PCR, which eliminates the loss of DNA that traditionally occurs during DNA extraction and quantification. (See Exhibit 1 and “Trace DNA and the Steps of DNA Processing.”)
Laboratories in the United States already use direct PCR for reference samples where the DNA donor is known, but current federal quality assurance standards keep them from using direct PCR for forensic evidence.[1]The federal standards require all unknown forensic samples to undergo DNA quantification prior to amplification.
In 2016, the Organization of Scientific Area Committee for Forensic Science was formed to address the lack of discipline-specific forensic science standards. It called for an assessment of the standard that recommends extraction and quantification during DNA processing of all evidence types, even those with low DNA yields. Soon after, NIJ’s Forensic Science Research and Development Technology Working Group identified a need for enhanced DNA evidence collection techniques that maximize DNA recovery. This group also identified a need for comprehensive studies that provide practical data about DNA transfer and persistence, or more simply, how DNA evidence gets on items and how long it tends to remain there.
Direct PCR Outperformed Standard PCR for Many Trace DNA Items
NIJ-funded researchers, led by Jonathan Davoren of Bode Technology, sought to reassess the current FBI guidelines for processing unknown forensic samples. They also assessed the operational requirements needed to maximize DNA recovery using direct PCR, which produced practical data about trace DNA evidence collection and analysis.
Davoren’s team sought to better understand:
- How different types of materials work with the direct PCR process by comparing evidence collection results from a variety of items using direct PCR and standard PCR. (Exhibit 2)
- How well direct PCR works (a) after items have been stored for various periods of time and (b) when items are resampled after their initial analysis, by examining the quality of the DNA profiles produced.
First, researchers collected DNA from11 items and processed them using the standard PCR (extraction, quantification, and amplification) and direct PCR methods (no extraction or quantification). The researchers then assessed the quality of the DNA profile based on whether the profile met the criteria to be uploaded into CODIS (the CODIS eligibility success rate).[2]
The direct PCR method produced higher quality DNA samples than standard PCR for trace DNA found on certain items, such as the plastic slides, polyester, metal tool, handgun grip, wood handle, and foam cup (Exhibit 3). However, the direct PCR method produced lower quality DNA samples than the standard PCR method for other items, including the vinyl shutter, denim, 100% wool, concrete bricks, and cartridge casings (Exhibit 4).
| Sample type | Standard PCR Success Rate (%) | Direct PCR Success Rate (%) |
|---|---|---|
| Plastic Slide Buccals | 82 | 97 |
| Polyester | 63 | 88 |
| Metal Tool | 56 | 69 |
| Handgun Grip | 33 | 54 |
| Wood Handle | 37 | 50 |
| Foam Cut | 13 | 19 |
| Plastic Slide Fingerprints | 7 | 14 |
| Sample type | Standard PCR Success Rate (%) | Direct PCR Success Rate (%) |
|---|---|---|
| Vinyl Shutters | 35 | 25 |
| Denim | 100 | 0 |
| 100% Wool | 100 | 0 |
| Concrete Blocks | 44 | 0 |
| Cartridge Casings | 6 | 0 |
To examine the effects of time and storage on the collection of trace DNA using direct and standard PCR, they conducted a time study on items that had been stored for three and six months. The direct PCR results did not differ significantly from those produced when items were processed immediately after collection, and they were comparable to, or better than, standard PCR for all items and time points, excluding 100% wool at six months, and concrete bricks and cartridge casings at all time points.
Finally, the group studied the effects of re-sampling items by reprocessing samples using the same method with which they had been previously processed (either direct or standard PCR). In general, the resampling was unsuccessful. The wood tool handle was the only item with which direct PCR was somewhat successful, which suggests that the texture of unfinished wood may help retain DNA. Resampling was less successful on samples processed with direct PCR compared to those processed with standard PCR for plastic slides and cartridge casings at zero months, 100% wool at six months, and concrete bricks at all time points.
Caution Regarding Contamination and Re-analysis
The researchers cautioned that the sensitivity of direct PCR processing resulted in a small amount of contamination with foreign DNA (6% of samples), which they attributed to both known sources, introduced prior to and during lab processing, and unknown sources. Moreover, the direct PCR process consumes the portion of the sample used. This means that there is no DNA extract created and no sample remaining for re-analysis unless it is purposely set aside.
Informing Federal Guidelines and Practice
The direct PCR method for trace DNA processing can produce complete DNA profiles in less than three hours and save labs approximately three to four hours of hands-on time and 25% in reagent costs. Although the process was not effective across all types of items, it was an improvement over standard DNA processing of trace DNA for seven of the 11 items tested.
The results of this research inform the reevaluation of federal guidelines and could simplify sample collection and submission guidelines for forensic laboratories. If a revision of federal guidelines includes direct PCR for forensic analyses, these results suggest that investigators should ensure that enough of the original sample remains for re-testing and limit the types of items analyzed to those shown to produce high-quality DNA using direct PCR.
Sidebar: Trace DNA and the Steps of DNA Processing
What is trace DNA? Trace DNA refers to small amounts of DNA left behind at a crime scene, such as a few cells left on objects. It can be challenging to obtain high-quality DNA profiles from this type of sample.
| Step | Description |
|---|---|
| DNA Extraction | A method to purify DNA from a sample using physical or chemical methods to separate DNA from cell membranes, proteins, and other cellular components. |
| DNA Quantification | A procedure commonly performed to measure the average amount of human DNA present in a sample, as well as its general quality and purity. For best results, DNA with a high level of purity and in specific amounts is needed. |
| DNA Amplification | The process that occurs during the polymerase chain reaction (PCR),where small segments of genetic material in a sample are copied multiple times. Sometimes called "molecular photocopying.” Scientists use this to target select segments of interest, thereby creating the amount necessary for forensic analyses. This process is often followed by capillary electrophoresis (also called DNA sequencing), to determine the unique “genetic fingerprint” of a sample. |
For more Stability and Persistence of Touch DNA for Forensic Analysis.
About This Article
The work described in this article was supported by NIJ grant number 2019-DU-BX-0009, awarded to Bode Cellmark Forensics, Inc. This article is based on the grantee report “Improving Results from Touch DNA Evidence with Optimized Direct PCR Methods” (pdf, 27 pages), by Jonathan M. Davoren, Abigail S. Bathrick, and Anna C. Salmonsen.