The investigation following an explosion or bombing plays a vital role in uncovering the truth about the incident. Criminal justice practitioners often need to build cases and attribute involvement in a crime by locating and using trace amounts of evidence remaining at the scene. The evidence recovered can be critical in identifying, charging, and ultimately convicting the person who perpetrated the crime to prevent further attacks.
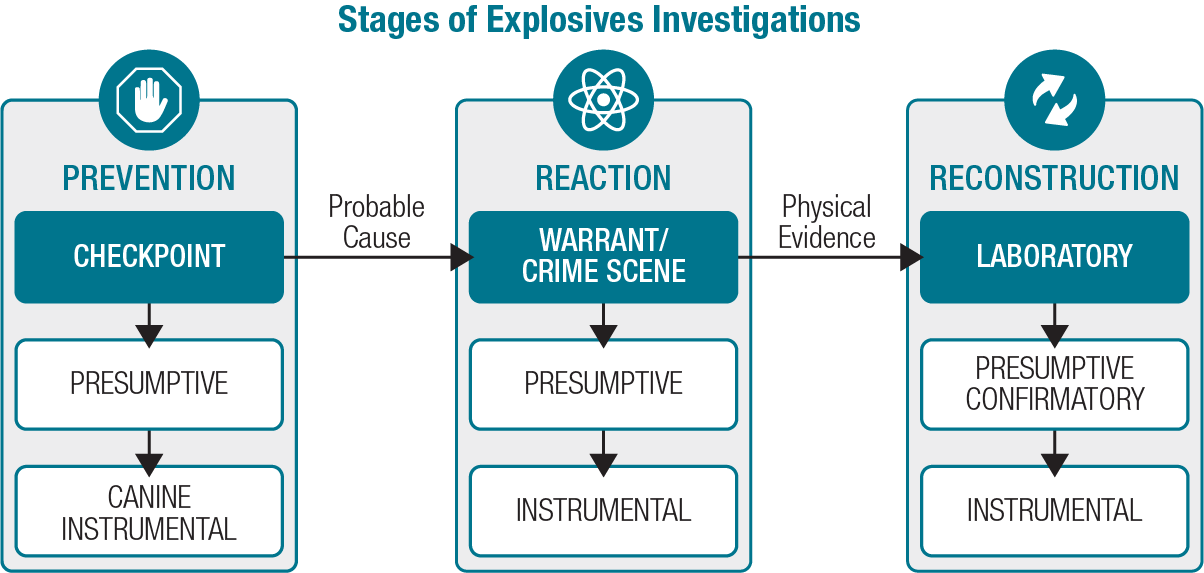
Investigations around explosives are inherently multidisciplinary, involving law enforcement officers and various specialists including scientists and engineers. Explosives investigations have three stages: prevention, reaction, and reconstruction of the incident (see exhibit 1). Investigators characterize each stage by where it tends to occur — at security checkpoints, during search warrants and at the crime scene, and in the forensic laboratory. During each stage, investigators can use different types of testing, such as presumptive field tests, explosives-detecting canines, portable instruments, and confirmatory testing with advanced laboratory instrumentation.
The National Institute of Justice (NIJ) has a long history in helping to advance the forensic technology used in the aftermath of terrorist attacks. This article highlights findings from two NIJ-funded projects related to the reconstruction phase of explosives investigations. The first project, funded in fiscal year 2017, examines the application of a new analytical tool for explosives traces: gas chromatography-vacuum UV spectroscopy (GC-VUV). The second project, funded in fiscal year 2018, looks at whether isotopic signatures of the residues at a blast site can link an explosive charge to its manufacturing source. Both projects help expand the toolkit investigators have for developing leads from these challenging crime scenes.
The Role of Chemical Analysis in Explosives Investigations
Over the years, forensic chemists have used physical, microscopical, wet chemical, and instrumental methods to identify and compare evidence. Instrumental methods lie at the heart of the forensic examination of controlled substances, ignitable liquids, explosives, and many other forms of physical evidence. This stems from the ability of modern chemical instrumentation to measure substances with appropriate sensitivity, selectivity, and specificity. However, forensic chemists must understand and consider the strengths and weaknesses of any given instrument.
In general, any instrumental method of analysis for explosives investigations must possess three main qualities:
- Sensitivity is the extent to which the instrument responds to low levels of the substance being analyzed (or the analyte). It is commonly defined as the slope of the calibration curve for an analyte. A highly sensitive method increases the chances of detecting an analyte if it is present, even at low levels, and avoiding false negatives. Highly sensitive methods also decrease the need for sample pre-concentration, which often involves applying heat and a flow of inert gas to a sample or other methods to increase the concentration of the analyte.
- Selectivity is the ability of the instrument to respond to an analyte that is present in a complex mixture, including compounds that have similar chemical structures to the analyte. Instruments often achieve this by chemically separating the mixture of compounds so that they can analyze each compound on its own without other components of the mixture interfering. Increased selectivity allows researchers to analyze highly complex samples without extensive preliminary clean-up steps, which saves time and money. In addition, selective methods can detect an analyte even in the presence of interferences or compounds that may mask the analyte.
- Specificity is the ability of the instrument to unambiguously identify the analyte. Several techniques are capable of discerning differences between similar analytes based on small structural differences. Increased specificity eliminates ambiguity when identifying an analyte. This is particularly valuable when the protocols for a given evidence type rely on the unambiguous identification of specific compounds to reach a scientific and legal opinion. For example, chemists must identify nitroglycerin in post-blast debris in order to infer that double-base smokeless powder (which contains large amounts of nitroglycerin) was the original explosive.
In forensic analyses, all three of these factors come into play. Sensitivity is important because many evidence types contain the analyte of interest at trace levels (for example, post-blast explosives and ignitable liquid residues). Selectivity is important because most items of evidence are messy and can contain many interferents. And specificity is crucial as laboratory results must be reliable and probative for courts to admit them as evidence.
A New Tool for Explosives Analysis
A relatively recent development in the field of instrumental chemical analysis couples a vacuum UV (VUV) spectrometer to a gas chromatograph (GC).[1] Its application to explosives analysis by NIJ-funded researchers at Indiana University is even newer.
A sample separated by GC may contain hundreds of compounds. The sample is vaporized and travels through a long, thin, coated tube. Each component in the mixture has its own affinity for the walls of the tube compared to the carrier gas, which affects the time it takes to travel through the column. As a result, over the course of a few minutes, the mixture separates — the compounds with low affinity for the column walls emerge first, and the compounds with high affinity emerge last. This allows the instrument to analyze each component of the mixture separately.
VUV spectroscopy can serve as the detector for the GC column. The analytes emerging from the column pass into a flow cell, and the spectrometer measures their ultraviolet absorption in real time. All organic compounds absorb in the VUV (roughly 100-200 nanometers), and small changes in chemical structure can result in significant changes in the VUV spectrum.[2]
The researchers at Indiana University found that the sensitivity of GC-VUV will differ for various analytes and under various conditions. In general, some materials require only picograms (10-12 grams) to meet their detection limits. For explosives, the method can readily detect concentrations in the low parts-per-million (0.0001%) range.[3]
The selectivity of GC-VUV comes from the fact that certain functional groups will reliably absorb in distinct regions of the VUV spectrum.[4] By selectively filtering these regions, chemists can cancel out interferences.
Researchers have demonstrated the specificity of GC-VUV under some conditions using statistical methods.[5] For example, Cruse and Goodpaster showed that the temperature of the flow cell can strongly influence the VUV spectra of some explosives, yielding complex and highly specific results.[6] Reavis and Goodpaster have also successfully identified and quantified intact smokeless powder particles from pipe bomb debris.[7]
Future work in this area should include attempts to increase GC-VUV’s sensitivity. This is necessary because post-blast residues of high explosives typically yield extracts with concentrations in the parts-per-billion range. It will also be crucial to increase specificity by increasing the level of spectral detail measured in the VUV.
Post-Blast Explosives Attribution
In crimes involving explosives, examining the explosive material itself is preferable when attempting to attribute the source of the device. However, this is not always straightforward. It is sometimes challenging for forensic science practitioners to analyze the residues collected after the blast because the environment may have been contaminated, the amount of explosive remaining after detonation may be too low, and the useful chemical signatures may degrade. These factors limit the analytical methods that they can apply. Currently, there is no established method for forensic science practitioners to link an explosive charge to its manufacturing source by studying the chemical signatures detected in post-blast trace residues.
With support from NIJ, researchers at the Massachusetts Institute of Technology Lincoln Laboratory collaborated with statisticians at South Dakota State University to determine whether isotopic and chemical signatures that might link explosive materials to their manufacturing sources remain preserved after detonation. This includes whether investigators can recover these explosive materials from a blast site, measure them at a detectable level, and match them to pre-blast signatures.[8]
The team conducted field detonations of several commonly encountered explosives materials, including RDX, TNT, and ammonium nitrate-aluminum (AN-AL). They designed the tests to be as operationally relevant as possible by using an open outdoor environment. For each detonation, they collected trace residues using methods relevant to scenarios that post-blast investigators would encounter, including swabbing surfaces and extracting residues from soil.
Then the team processed the samples of post-blast residue and analyzed them to measure their isotopic and chemical signatures.[9] They used statistical analysis to compare these post-blast signatures to those from pre-blast samples to determine if they remained preserved after detonation.
In total, the team collected 108 post-blast samples and three pre-blast samples for each explosive type. They concluded that the results showed some consistency between pre- and post-blast explosive materials that could be relevant for source attribution. AN-AL yielded the most useful post-blast data.
One key limitation to the study was obtaining recoverable amounts of RDX and TNT. These high-order explosives result in detonations that consume all or nearly all the explosive material. Nonetheless, the research team concluded that the overall results show promise in the ability to detect and identify signatures for attribution in post-blast residues. They noted that this study provides the first step in developing a new investigative method to associate an explosives attack to a person suspected of committing the crime (through a manufacturer) to supplement current post-blast investigative methods.
Moving Forward
These projects show the promise and the challenges in applying advanced analytical methods to the complex aftermath of an explosion or bombing. Because these difficult trace samples challenge the limits of current technology, the field needs continued research and development to pave the way to the tools of the future. Eventually, those tools may help investigators extract more information from the scene: developing leads, identifying individuals suspected of committing the crime, and confirming the source of a device with greater confidence.
Explore NIJ’s full forensic science research and development portfolio.
About This Article
This article was published as part of NIJ Journal issue number 285. This article discusses the following awards:
- “Coupling Gas Chromatography (GC) and Vacuum Ultraviolet (VUV) Spectroscopy for Forensic Applications,” award number 2017-R2-CX-0018
- “Post-Blast Explosives Attribution,” award number DJO-NIJ-19-RO-0002-2
Opinions or points of view expressed in this document represent a consensus of the authors and do not necessarily represent the official position, policies, terminology, or posture of the U.S. Department of Justice on domestic violent extremism. The content is not intended to create, does not create, and may not be relied upon to create any rights, substantive or procedural, enforceable at law by any party in any matter civil or criminal.