Home | Glossary | Resources | Help | Contact Us | Course Map
Aviso de archivo
Esta es una página de archivo que ya no se actualiza. Puede contener información desactualizada y es posible que los enlaces ya no funcionen como se pretendía originalmente.
Sodium Rhodizonate Test
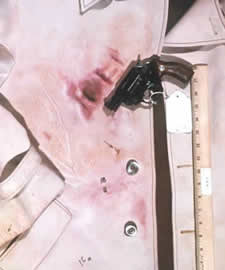
The Sodium Rhodizonate Test is a chemically specific chromophoric test for the presence of lead in any form, including vaporous lead (smoke), particulate lead, lead in primer residues (e.g., lead azide or lead styphnate), lead bullet, or shot pellet wipe. The presence of particulate lead is a random nonreproducible phenomenon dependent on many uncontrolled variables that may be caused by leading, metal fouling, or a dirty barrel at the time of discharge.
The presence of vaporous lead is very useful in that it typically is found at closer ranges. Although the Sodium Rhodizonate Test cannot be used to determine precise muzzle-to-target distances, it is possible to determine the maximum distance to which it will be deposited using the firearm and ammunition in combination.
The procedure can be applied to a number of surfaces, such as victim clothing, drywall, vehicle upholstery, curtains, carpet, etc. It is always performed after the Modified Griess Test and the Dithiooxamide Test to preclude potential chemical interference.
The objective of this test is to confirm the results of the Modified Griess Test. Information from this test may also assist in establishing a muzzle-to-target distance; lead is generally detected at closer distances. A positive result around the suspected bullet hole is consistent with passage of a bullet.
Chemistry
The chemistry of the Sodium Rhodizonate Test is comprised of the following process:
- The questioned area is sprayed directly with a saturated solution of sodium rhodizonate in distilled or deionized water.
- A buffer solution (pH 2.8) consisting of sodium bitartrate and tartaric acid in distilled or deionized water is then sprayed on the area.
Any pink reaction which results may indicate lead or a number of other possible heavy metals. The presence of lead may be confirmed through an additional step. - A dilute solution of hydrochloric acid is applied by spraying the area.
The color results are dependent on the degree of acidity and the presence of lead. If the pink area changes to a blue-violet color, the presence of lead is confirmed.
Reagents and Test Media
The following reagents and test media are required for the Sodium Rhodizonate Test:
- Saturated solution of sodium rhodizonate dissolved in distilled or deionized water
- Buffer solution of 2.8 pH consisting of sodium bitartrate and tartaric acid in distilled or deionized water
- Solution of hydrochloric acid (5 percent) in distilled or deionized water
- A known source of lead
Sodium Rhodizonate Solution
Add a sufficient amount of distilled or deionized water to a small amount of sodium rhodizonate in a small beaker to form a saturated solution that is approximately the color of dark tea. The solution is saturated if a slight amount of sediment remains in the beaker after stirring with a glass stirring rod. The solution is usually ineffective after one hour.
2.8 pH Buffer Solution
- Dissolve 29.3 grains (1.9 grams) of sodium bitartrate and 23.1 grains (1.5 grams) of tartaric acid per 100 mL of distilled or deionized water. Heat on a hot plate while agitating with a magnetic stirrer to create the solution quickly.
- Store in a sealed container labeled according to laboratory protocol. The shelf life of the solution is limited.
Hydrochloric Acid Solution
- Combine 5 mL of concentrated hydrochloric acid with 95 mL of distilled or deionized water. Carefully pour the acid into the water to preclude spattering of acid.
- Store in a sealed container labeled according to laboratory protocol.
Test Procedure
This test procedure involves applying chemical reagents using aerosol sprays. This can be readily accomplished using cans of compressed gas. All spraying should be performed in a chemical fume hood.
The Sodium Rhodizonate Test is carried out as follows:
- Confirm performance of all preliminary examinations and tests.
The Modified Griess Test and the Dithiooxamide Test must be performed before this test. - Place a test mark on the item to be tested.
Using a known piece of lead, place the mark well away from any areas to be tested.
(Note: Apply the following steps to the test mark first to ensure the reagents are reacting properly and then apply to the questioned area.) - Position the area to be tested.
Place the test mark or suspected bullet hole face up on a clean uncontaminated surface. - Perform color test.
- Spray the test area with the saturated solution of sodium rhodizonate.
- Spray the same area with the buffer solution.
This solution eliminates the yellow background color caused by the sodium rhodizonate, establishes a pH of 2.8, and displays a pink color in the presence of lead and some other heavy metals. - Spray the same area with the hydrochloric acid solution.
The pink color fades leaving a blue-violet color, indicating the presence of lead.
This result can fade quickly; observations should be photographed and documented promptly.
See the YouTube Terms of Service and Google Privacy Policy
Additional Online Courses
- What Every First Responding Officer Should Know About DNA Evidence
- Collecting DNA Evidence at Property Crime Scenes
- DNA – A Prosecutor’s Practice Notebook
- Crime Scene and DNA Basics
- Laboratory Safety Programs
- DNA Amplification
- Population Genetics and Statistics
- Non-STR DNA Markers: SNPs, Y-STRs, LCN and mtDNA
- Firearms Examiner Training
- Forensic DNA Education for Law Enforcement Decisionmakers
- What Every Investigator and Evidence Technician Should Know About DNA Evidence
- Principles of Forensic DNA for Officers of the Court
- Law 101: Legal Guide for the Forensic Expert
- Laboratory Orientation and Testing of Body Fluids and Tissues
- DNA Extraction and Quantitation
- STR Data Analysis and Interpretation
- Communication Skills, Report Writing, and Courtroom Testimony
- Español for Law Enforcement
- Amplified DNA Product Separation for Forensic Analysts