Aviso de archivo
Esta es una página de archivo que ya no se actualiza. Puede contener información desactualizada y es posible que los enlaces ya no funcionen como se pretendía originalmente.
Home | Glossary | Resources | Help | Contact Us | Course Map
Most quantitative PCR kits monitor the increase in fluorescent signal throughout the PCR cycling process. The change in fluorescence is monitored between samples and standards so that a comparison can be made.
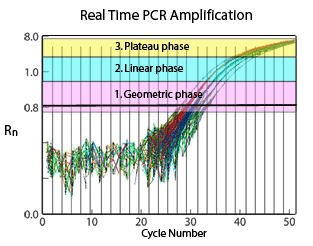
The amplification process can be summarized in three consecutive phases:
- Exponential/ Geometric amplification
- Linear amplification
- Plateau region
Read more about the PCR process in course: Crime Scene and DNA Basics for Forensic Analysts.
During the exponential phase of the PCR process, the reaction results in a theoretical doubling of amplicons with each cycle. While the ideal efficiency is not achieved, the doubling is close to 100%, yielding a consistent relationship of input DNA to product.13
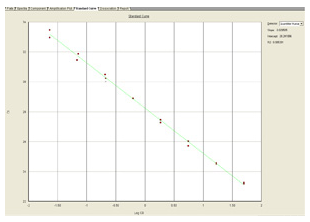
At the beginning of the exponential phase, the baseline is determined by measuring the background fluorescence signal (noise). This baseline establishes the threshold. During the amplification process of a sample, the point (in terms of amplification cycles) in which the level of fluorescence exceeds the threshold is referred to as the Cycle Threshold (CT) . The CT value is lower for a sample with a higher initial concentration and is higher for a lower-concentration sample.
The standard curve is a regression line that is derived from the CT values of the standards plotted against the log of the concentration of the standards. The concentration of each sample is determined by comparing its CT value against the standard curve.
The consumption of one or more PCR reagents during the amplification process will impede the efficiency, causing the onset of the linear phase. Product amplification slows down yielding an inconsistent ratio of input DNA to product. Therefore, the linear phase is not commonly used in the data analysis process.
The final amplification phase signifies the depletion of critical reagents and is known as the plateau region. During this phase, the amplification process ceases.
Quantitation Methods and Kits
There are several quantitative PCR methods currently is use. Quantitation kits are available through commercial sources or may be developed in-house. The National Institute of Standards and Technology (NIST) has developed a new standard that can be used as a quality control measure to assess the accuracy of quantitation results.
Learn more about SRM 2372-Human DNA Quantitation Standard from the NIST STRBase Web site.
Additional Online Courses
- What Every First Responding Officer Should Know About DNA Evidence
- Collecting DNA Evidence at Property Crime Scenes
- DNA – A Prosecutor’s Practice Notebook
- Crime Scene and DNA Basics
- Laboratory Safety Programs
- DNA Amplification
- Population Genetics and Statistics
- Non-STR DNA Markers: SNPs, Y-STRs, LCN and mtDNA
- Firearms Examiner Training
- Forensic DNA Education for Law Enforcement Decisionmakers
- What Every Investigator and Evidence Technician Should Know About DNA Evidence
- Principles of Forensic DNA for Officers of the Court
- Law 101: Legal Guide for the Forensic Expert
- Laboratory Orientation and Testing of Body Fluids and Tissues
- DNA Extraction and Quantitation
- STR Data Analysis and Interpretation
- Communication Skills, Report Writing, and Courtroom Testimony
- Español for Law Enforcement
- Amplified DNA Product Separation for Forensic Analysts